
What are CHEMICAL CHANGES and PROPERTIES?
CHEMICAL PROPERTIES:
- Definition (on top of flap)– Properties that can be observed during or after a chemical reaction. The substance is changed to a NEW substance.
- Examples (under flap)– How easily something catches fire (flammability), decomposition (if something will rot and break down), reactivity with air or water (rust is an example), how toxic it might be.
CHEMICAL CHANGES-
- Definition (on top of flap)–
- A substance changes into a new substance with different properties.
- Substances present at the beginning of the change are NOT present at the end.
- Can’t or is very difficult to undo!
- Evidence of a Chemical Change (under flap)- http://www.classzone.com/vpg_ebooks/ml_sci_gr8/accessibility/ml_sci_gr8/page_155.pdf
- Different signs include
- an odor is produced
- light or smoke is seen
- temperature change- it takes in or gives off heat
- production of gas bubbles
- color change (like rusting)
- Different signs include
WATCH
- WATCH
- Bozeman Science: Physical and Chemical Changes– http://www.bozemanscience.com/physical-chemical-changes/
- Physical vs Chemical Changes– https://www.youtube.com/watch?v=hcunQqbNEMQ
- Physical vs Chemical Changes with Mark and Molly– https://www.youtube.com/watch?v=M8tyjwB42X4
- Crash Course: Kitchen Chemical Changes– https://www.youtube.com/watch?v=37pir0ej_SE
- Physical and Chemical Changes for Kids– https://www.youtube.com/watch?v=BgM3e8YZxuc
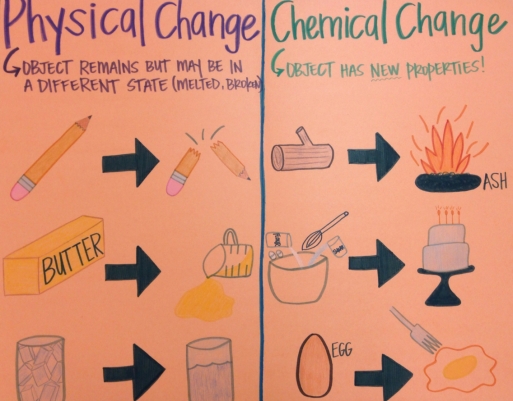
EXAMPLES OF PHYSICAL AND CHEMICAL CHANGES
- Chemical vs Physical Changes- https://www.learner.org/courses/essential/physicalsci/session4/closer1.html
- Chemical Reactions/Changes
- 10 Examples of Everyday Chemical Reactions- http://chemistry.about.com/od/chemicalreactions/ss/10-Examples-of-Chemical-Reactions-in-Everyday-Life.htm#showall
- Chemical Change Examples- http://www.buzzle.com/articles/chemical-change-examples.html
- Examples of Chemical Changes- http://www.chemistryforkids.net/reactions/what-is-a-chemical-change
- Physical Changes
- 10 Examples of Physical Changes- http://chemistry.about.com/od/matter/a/10-Physical-Change-Examples.htm
- Physical Change Examples- http://www.softschools.com/examples/science/physical_changes_examples/170/
LET’S PLAY (Use Chrome)
- Choose: Is it a physical or chemical change? http://vital.cs.ohiou.edu/steamwebsite/downloads/ChangeLab.swf
- Glencoe Physical and Chemical Change Virtual Lab- http://www.glencoe.com/sites/common_assets/science/virtual_labs/E03/E03.html
- Reversable (physical) and Irreversable (chemical) Changes, BBC- http://www.bbc.co.uk/schools/scienceclips/ages/10_11/rev_irrev_changes_fs.shtml
LAB INFORMATION RESOURCES
Baking Soda
- Chemical Properties:
- Exploritorium: scroll down the page for information on how this works and other questions to ask.
- Science Bob: has reaction information and things to test.
